Hearing Loss and Central Auditory Processing
55
Learning Objectives
Be able to discuss the impact of exposure to loud noises related to this condition.
Be able to describe the impact on behavioral thresholds.
Hidden hearing loss wasn’t reported before 2009 (Kujawa et al, J. Neurosci 19:14077-14085) but it is rapidly garnering broad attention. Even when the hair cells are not damaged and behavioral thresholds recover completely after loud noise exposure, 50% of the synapses are gone! But hearing thresholds as measured clinically and reported in audiograms are still the same. The technical name for hidden hearing loss is cochlear synaptopathy.
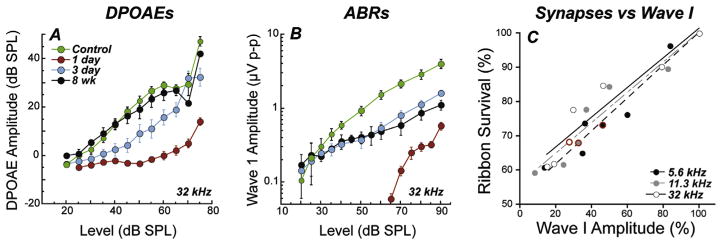
To explore this finding of noise-induced primary neuropathy further, and to clarify interpretation, the observations were repeated for an exposure that produced only temporary threshold shift (TTS) in fully adult animals (Kujawa and Liberman, 2009). In this work, mice from the same inbred strain were exposed to a band of noise placed in the region of best threshold sensitivity. The noise was titrated in level and duration to produce a large, acute threshold shift (30–40 dB at 24 h), but one that recovered by 2 weeks, without hair cell loss. Immunostained cochlear whole mounts and plastic-embedded sections (Fig. 6.5.1), imaged by confocal and conventional light microscopy, were assessed to quantify hair cells, cochlear neurons, and synaptic structures providing the communication conduits. Hair cell-based distortion product otoacoustic emissions (DPOAEs) and neural-based auditory brainstem responses (ABRs) or compound action potentials (CAPs) of the auditory nerve were used to assess the peripheral consequences of the noise on function (Fig. 6.5.1).
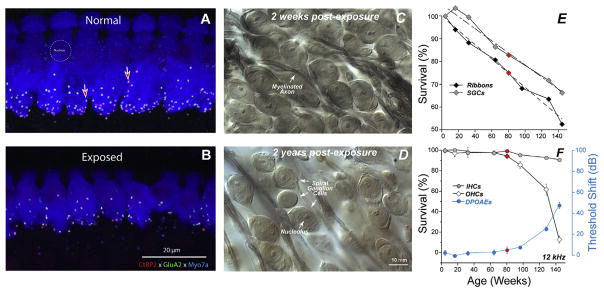
Presaging the ganglion cell losses, results of these studies revealed an acute loss of synapses between IHCs and the peripheral terminals of the spiral ganglion neurons that contact them (Kujawa and Liberman, 2009). Although thresholds recovered, by design, and no hair cells were lost, IHC synaptic losses were greater than 40% in basal cochlear regions, when assessed 24 h post noise, and were stable 2 and 8 weeks later. Losses were proportional in magnitude and cochlear location to the SGC loss observed in the previous series, suggesting that this interruption of IHC-to-neural communication set the stage for neurodegeneration.
For an additional explanation about hidden hearing loss, take a peak at the video link here and included below.
CC LICENSED CONTENT, SHARED PREVIOUSLY
Cheryl Olman PSY 3031 Detailed Outline
Provided by: University of Minnesota
Download for free at http://vision.psych.umn.edu/users/caolman/courses/PSY3031/
License of original source: CC Attribution 4.0
National Center for Biotechnology Information, “Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms” by M. Charles Liberman and Sharon G. Kujawa
Provided by: Hearing Research
URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438769/
License: CC BY 4.0
References:
Kujawa, S. G., & Liberman, M. C. (2009). Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. Journal of Neuroscience, 29(45), 14077-14085.
Liberman, M. C., & Kujawa, S. G. (2017). Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hearing research, 349, 138-147.

