There are a number of ways to deliver DNA (or other genetic information) to neurons including transfection, electroporation, biolistic delivery, and nanoparticles, but within an intact biological system like the brain is challenging. Luckily, neurons are very easily infected by viruses (see Unit 1). Neurobiologists will, therefore, package genetic material that they want to be expressed within specific groups of neurons inside different types of viruses (Figure 1.).
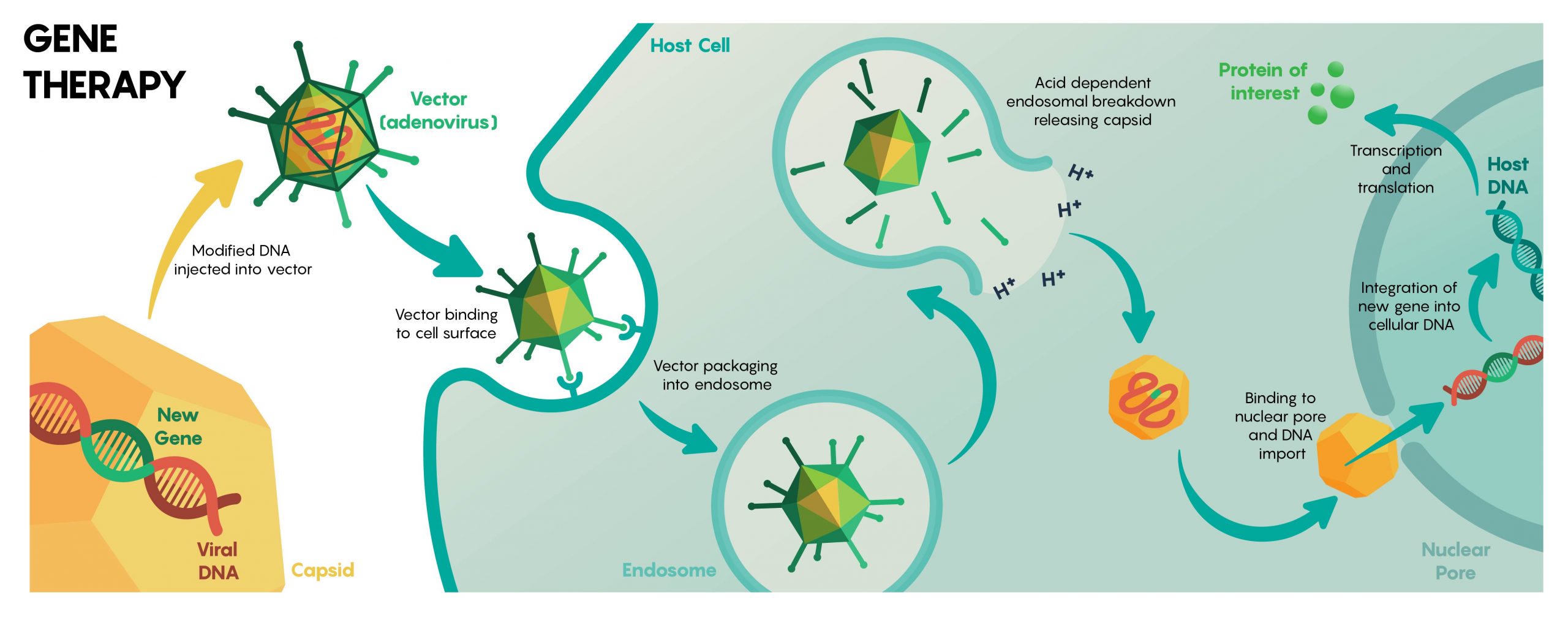
In fact, as molecular neurobiologists, we often have a number of different viral vectors that we can use to our benefit. Some viruses for example package only a small amount of genetic material (ie. AAV) while others like HSV will be able to package up to 150 kb worth of genetic material (and this is a lot) into the virus. Speed of expression and how long the expression of the recombinant genetic material will persist for, are often considerations on the choice of a viral vector (see Table 1).
| Properties of recombinant viral vectors useful for gene delivery in the adult nervous system | |||
|---|---|---|---|
| Adeno-Associated Virus (AAV) | Lentivirus | Herpes Simplex Virus (HSV) Amplicon | |
| Genetic material | Single-stranded DNA | RNA | Double-stranded DNA |
| Capacity for genetic material | ~5 kilobases | ~8 kilobases | ~150 kilobases |
| Speed of expression | Weeks | Weeks | Days |
| Duration of expression | Years | Years | Weeks to months, but elements can be added for persistent expression |
One technical consideration that is often overlooked – how are viruses containing the gene of interest that we hope to express in neurons generated? If viruses aren’t truly alive, then how does this work? We often use a cell line, like the HEK293 cell, which is a non-neuronal cell line. We transfect three separate plasmids into the HEK293 cells in culture. Two of these plasmids are viral (pVSV-G and packaging plasmids for example for lentiviruses), and the last one is the construct that we want to specifically deliver. Figure 2 shows the workflow for the production of lentiviruses (upper panel) and AAV viruses (lower panel). In both cases, viral particles are released from the transfected HEK293 cells and the virus is collected from the cells and spun on a gradient using ultracentrifugation and a final purification step. The purified fluid now contains a live virus that can infect neurons.


