Myelin is produced from the cell membranes of either Schwann cells (for neurons in the PNS) or oligodendroglia/oligodendrocytes (for neurons in the CNS). As such, myelin is enriched with membrane lipids and proteins that are found within these cells. Normally myelin functions to increase nerve cell conduction by increasing the biophysical property of the membrane resistance (i.e. a neuron cell membrane’s leakiness). Myelin reduces membrane leakiness by preventing open channels and as a result, increasing how far a single electrical impulse within the axon will travel. Importantly this also increases how quickly an action potential will travel down an axon – myelin greatly increases the conduction velocity of a neuron (Figure 1.).
Myelin helps neurons to cheat
Various factors help to determine how fast an action potential travels down an axon (called the conduction velocity). The biophysical features of a neuron including axonal diameter and membrane leakiness, help scientists to determine what is known as the length or space constant (lambda or λ) for each neuron. Why is this λ value important? λ is directly proportional to the conduction velocity – so the factors that determine λ also determine a neuron’s conduction velocity. Let’s not forget –speed thrills within the CNS and brain, and the faster a signal gets there, the more the brain likes it.
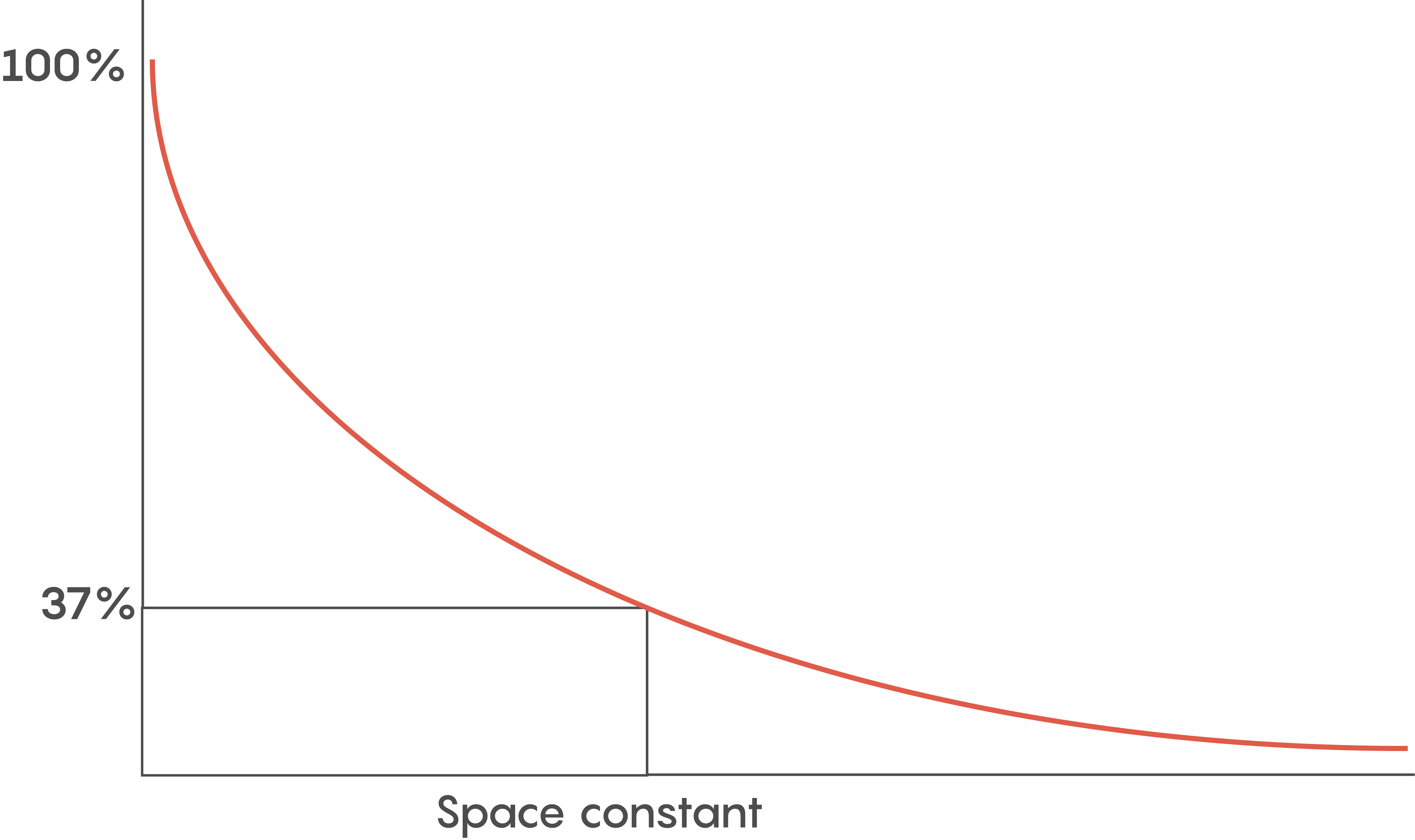
Another reason for myelination includes having a lot of neurons so space is a premium. Although the length constant is inversely related to the axonal diameter (i.e. bigger diameter axons have bigger length constants), we need to pack as many neurons into a small space as possible. So within the brain, faster communication means myelination is key.
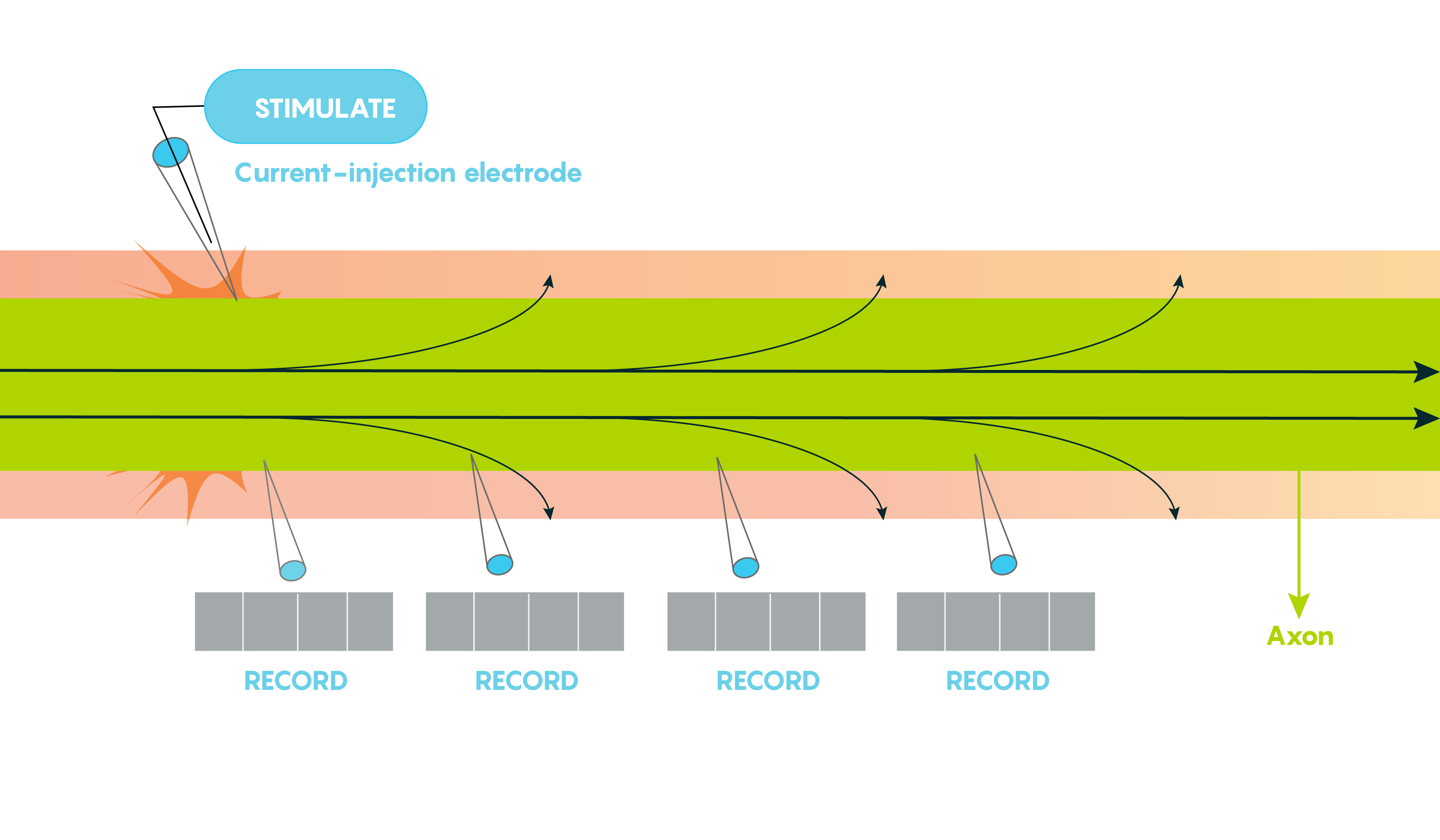
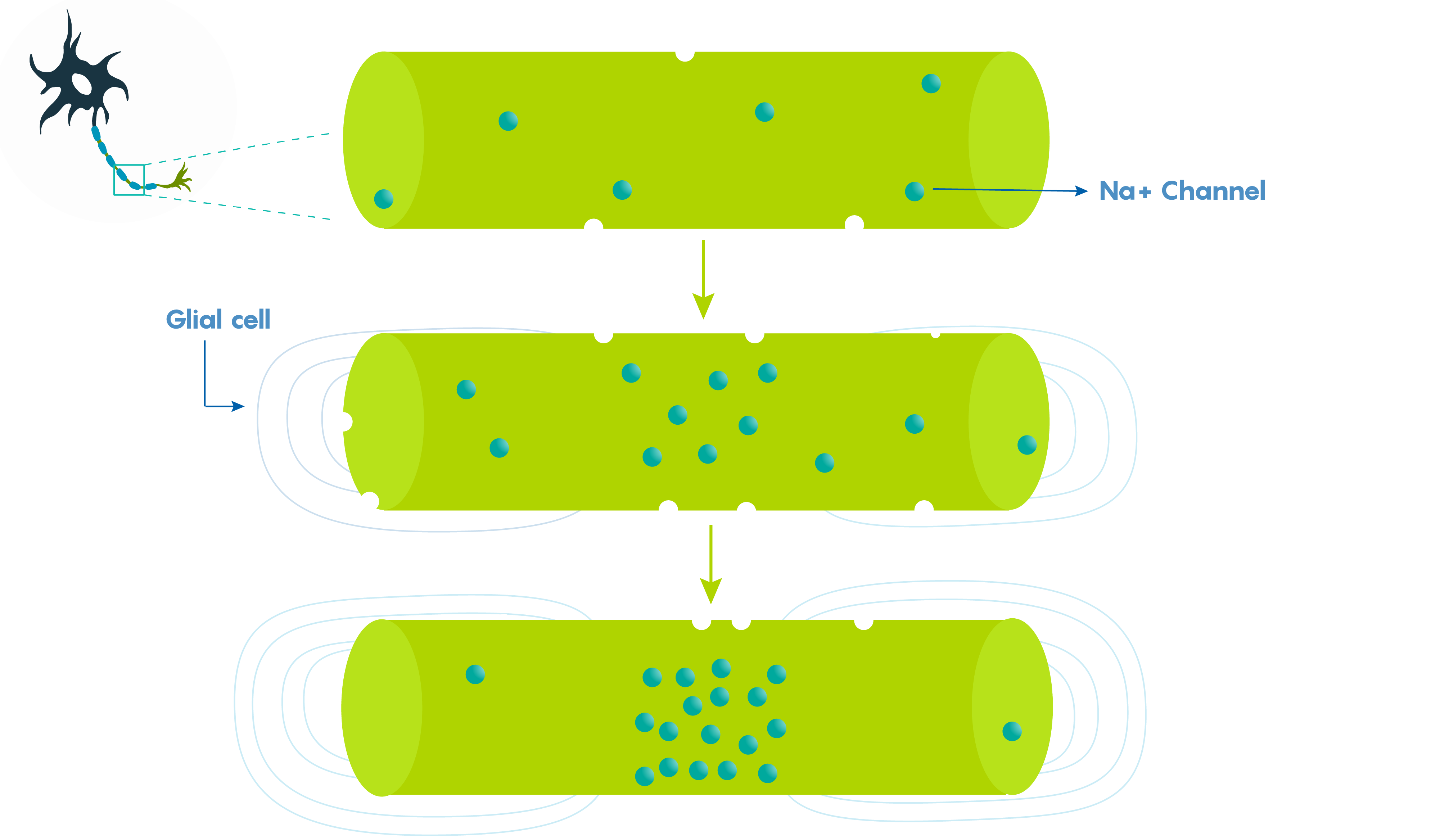
Large vertebrate nerve fibres are wrapped in myelin sheaths formed by central oligodendroglial cells or Schwann cells in the periphery but the myelin only wraps the axon at specific locations. Myelin is interrupted at regularly spaced intervals or nodes. This helps the neuron because during action potential propagation the excitatory signal jumps from one node to the next, and we call this saltatory conduction which is much faster than passive electronic spread. In addition, myelin also helps Rm (leakiness or membrane resistance) because it only directs voltage-sensitive Na+ channels to highly concentrated areas known as the nodes of Ranvier (Figure 2.). So in many ways, myelin helps the neuron “cheat’ by limiting leakiness (i.e. open channels to specific areas). As shown in Figure 2., Before glial ensheathment, sodium channels are distributed uniformly, and at low density. At the time of glial ensheathment but before the formation of compact myelin, loose clusters of sodium channels develop at sites that will eventually become nodes. Following the formation of compact myelin and mature paranodal axon-glial cell junctions, well-defined nodal clusters of voltage-gated sodium channels are established, and sodium channels are eliminated from the axon membrane beneath the myelin sheath.
Demyelinating diseases are prevalent – and in Canada, Multiple Sclerosis affects many
Demyelinating diseases remain one of the most common disorders where 1:500 to 1:1000 adult individuals in North America are affected, but these diseases tend to affect women more frequently than males. As the name suggests, within these diseases, the myelin described above is lost and the axons which previously have been myelinated no longer have myelin, changing their conduction velocities.
Multiple Sclerosis: Overview
Within this disease, the myelin is destructively removed from around the axon which slows down nerve impulses. As axons are demyelinated, these result in inflammatory patches called lesions and it is thought that this disorder is an autoimmune disease. As the disease progresses, oligodendrocytes and, ultimately, the axons themselves are destroyed. There is very compelling evidence that the destruction is caused by selective activation of the cellular immune system and inflammatory molecules.
Potential causes and theories
Currently, there is no known cause for multiple sclerosis although many different hypotheses have been put forward. Surprisingly, there are no known associative genes that have been implicated in causing multiple sclerosis and scientists believe there might be a complex interaction between genes and environmental factors to produce demyelination observed in multiple sclerosis. The most common theories about the cause(s) of multiple sclerosis include:
- Viral infection and resulting in autoimmune reaction where evidence from Experimental allergic encephalomyelitis mouse models suggest a strong link
- Genetic factors: inherited predisposition possibly through the immune system, although a mutation in a gene has not been discovered
- Environmental factor(s) which might work with genetics such as low vitamin D levels or smoking.
Although this is a well-characterized disorder, we still don’t know much about its causes.
Diagnosis, loss of function, and pathology
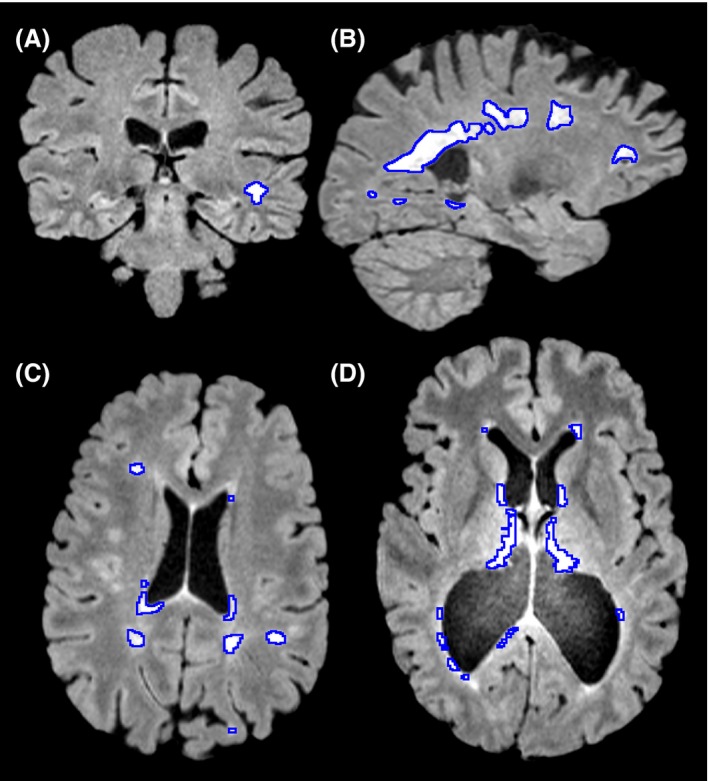
Typically an individual with demyelination in multiple sclerosis may have functional deficits that result from a reduced speed of conduction which might include loss of vision, different atypical sensations in the periphery and findings on an MRI that include hyperintensities, bright patches on structural MRIs (Figure 3.), around sites like the lateral ventricle, optic nerve, brainstem, spinal cord, cerebellum and other areas.
Multiple sclerosis also presents itself differently including having acute phases where the condition has been associated with relapsing/remitting where symptoms may not appear for periods of time and a chronic phase which is associated with progressive forms of multiple sclerosis where the individual progressively becomes worse and the symptoms become more severe.
Animal models of multiple sclerosis
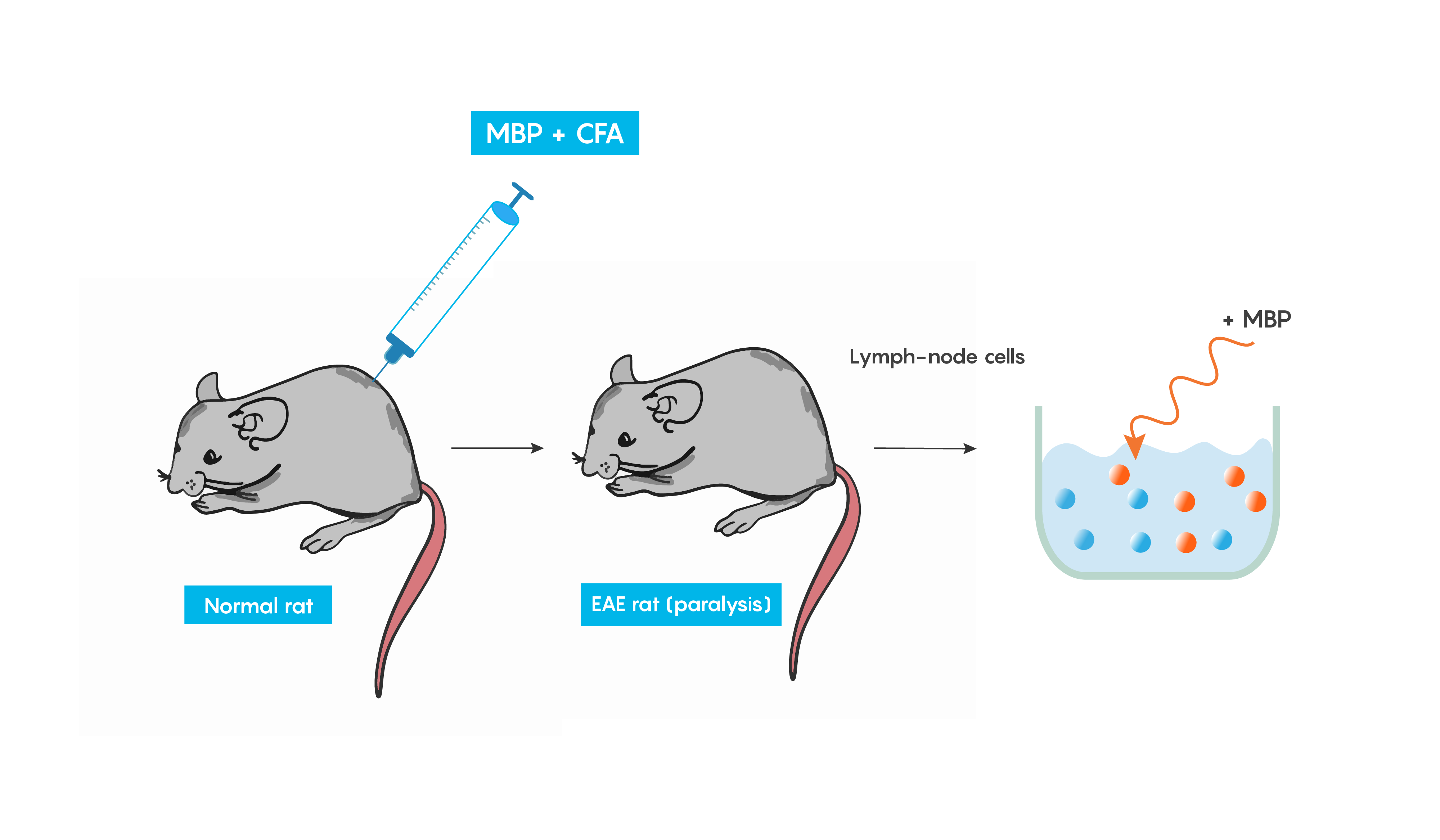
Experimental autoimmune encephalomyelitis (EAE): one of the best-studied models of autoimmune diseases. This model produces inflammation of both the brain and spinal cord. This is a good validity model for multiple sclerosis, as EAE is believed to be mediated by T cells and can be induced in many animal models following immunization with a myelin-specific protein such as myelin basic protein (MBP) or proteolipid protein (PLP) in Freund’s complete adjuvant (or CFA which helps to produce a specific immune response). Within weeks animals develop cellular inflammatory cell infiltration of the myelin sheaths of the central nervous system which then results in demyelination or paralysis (Figure 4.).
The cuprizone model of multiple sclerosis
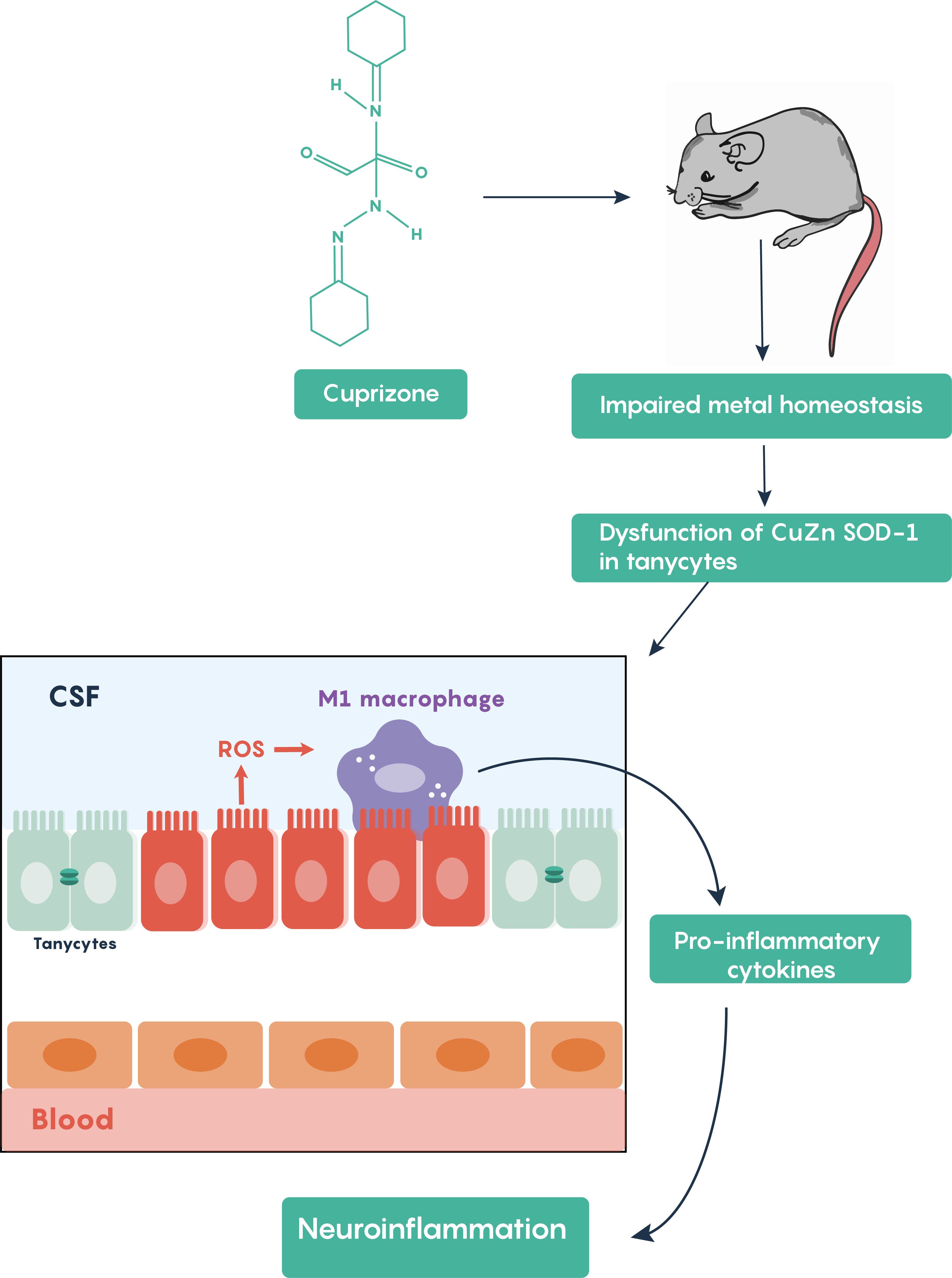
Cuprizone treatment is one of the most frequently used toxin-induced multiple sclerosis models. Other toxins such as lysolecithin or ethidium bromide require stereotaxic microinjections into brain areas and result in only localized focal demyelination but cuprizone models are advantageous in that oral administration of cuprizone produces global damage. Following cuprizone ingestion, the mouse often exhibits impaired divalent ion (Cu2+, Zn2+) homeostasis and results in the loss of Cu-Zn superoxide dismutase activity within the brain’s tanycytes resulting in the production of reactive oxygen species (ROS) which activate M1 macrophages in the brain to increase the production and release of pro-inflammatory molecules as highlighted in Figure 5.
Chapter Checkpoint: Complete the following quiz

